|
|
| Funding for this site is provided by readers like you. | |
|
|
|
|
|||||
|
|
|||||||
|
|
|
|
|
|
| |
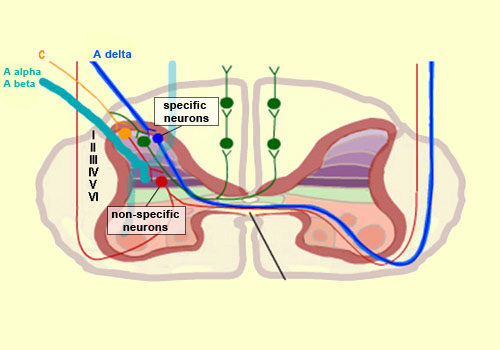
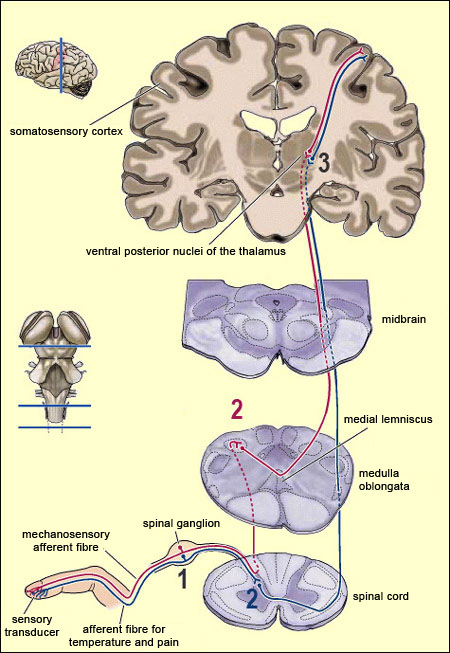
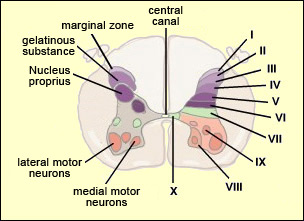
From the reception of a pain stimulus in the peripheral nervous system to the perception of pain in the brain, which generates various behaviours in response, the pain circuit follows several different, often redundant pathways. This is not surprising, considering how important pain is for the body’s integrity. These multiple nociceptive pathways all start in the same way. A pain signal coming from the skin, for example, first travels up a sensory nerve fibre composed of the axons of the T-shaped sensory neurons located in a spinal ganglion. These axons then enter the spinal cord, where they immediately divide and travel a short distance of one or two segments upward and downward in the spinal cord. They thus form what is called Lissauer’s tract before terminating in the external part of the dorsal horn. The particular areas of the dorsal horn where the various fibres (A alpha, A beta, A delta and C) synapse are not random: they map to a very specific spatial organization.
Thus the large-diameter, myelinated fibres (A alpha for touch and A beta for proprioception), which ascend directly into the ipsilateral dorsal column of the spinal cord, nevertheless also send out collateral axons that penetrate into the deepest layers of the dorsal horn (see box below), which make direct contact with the deep layers of the ventral horn. There the connections are made with the motor neurons that make the withdrawal reflex possible. Meanwhile, the smaller-diameter A delta and C fibres, which transmit pain, synapse on two main types of neurons that are also located in specific layers of the dorsal root of the spinal cord. The first type are known as specific nociceptive neurons, and their cell bodies are located in layers I and II. These neurons receive A delta and C fibres and hence are activated exclusively by mechanical and/or thermal nociceptive stimuli applied to the skin. The axons of these specific nociceptive neurons combine to form the neospinothalamic tract. The second type are known as non-specific neurons, and their cell bodies are located in layer V. These neurons respond preferentially but not exclusively to nociceptive stimuli. They are also known as convergent, polymodal, or wide-dynamic-range neurons, because they can be activated by fibres carrying non-nociceptive mechanical stimuli as well as by pain stimuli of tactile, muscular, or visceral origin. These neurons can also encode the intensity of the peripheral stimulus, by increasing their firing frequency as this intensity increases. Once this frequency exceeds a certain threshold, the message becomes nociceptive. The receiving field on the skin from which these non-specific neurons can be activated is larger than the field for specific neurons and also displays a sensitivity gradient: at the centre of the receiving field, all mechanical stimuli produce a nerve impulse, while at the edge, only nociceptive stimuli do so. The nerve impulses observed in non-specific neurons can therefore have an early component, due to the activation of the collaterals of the A alpha and A beta fibres, and then later components due to the activation of the A delta and C fibres in response to more intense stimuli. The fact that fibres of various diameters and of both visceral and tactile origin converge on these neurons also explains two distinct phenomena: projected pain, in which a pain in the internal organs is felt in an area of the skin, and the segmental control of pain by the activation of non-nociceptive afferent pathways.
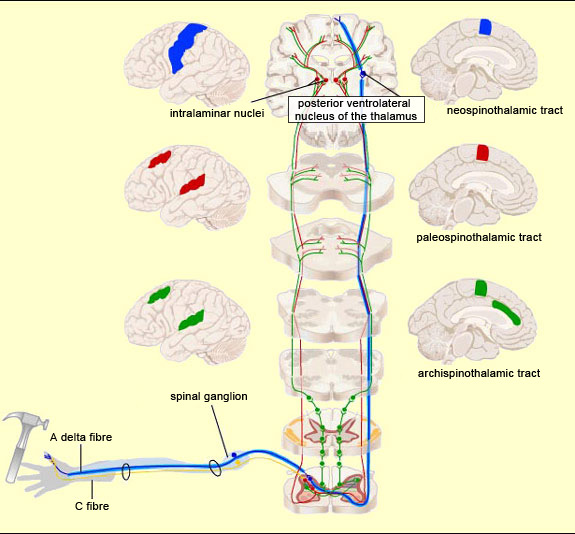
Thus pain signals are carried over three main extralemniscal pathways (as opposed to the lemniscal pathway, discussed below) that appeared successively in the course of evolution: the archispinothalamic tract, the paleospinothalamic tract, and the neospinothalamic tract.
For an animated version of this figure, see http://neuroscience.uth.tmc.edu/s2/ii7-2.html.
The pathway that appeared next in the course of evolution is the paleospinothalamic tract or paleospinothalamic pathway. (Note that this phylogenetic or evolution-based classification is not the only possible one—see first sidebar.) The paleospinothalamic tract is composed of small-diameter fibres that conduct nerve impulses slowly. Like the archispinothalamic tract, it has no somatotopic organization. It projects widely into the reticular formation at all levels of the brainstem, thus contributing to two important phenomena. The first is the maintaining of wakefulness in the central nervous system by the ascending reticular system. The second is the activation of certain nuclei in the brainstem that constitute the start of the descending pain-control pathways. The terminations of the paleospinothalamic pathway also continue into the intralaminar nuclei of the thalamus. The neurons of these nuclei (which are the third neurons in the afferent nociceptive pathways, after the neurons in the spinal ganglia and the neurons in the dorsal horn of the spinal cord) send projections into various cortical regions, including the frontal cortex, cingulate cortex, and insular cortex.
The neospinothalamic pathway or neospinothalamic tract, usually referred to simply as the spinothalamic pathway, is the most recent nociceptive pathway from an evolutionary standpoint and is found in higher mammals only. This is the pathway travelled by the rapid component of pain, which tells the brain about the nature of the painful stimulus (sting, burn, etc.) and its precise bodily location . This is also the pathway that transmits the sensation of temperature. The spinothalamic pathway consists of the axons of the specific neurons of the ventral horn of the spinal cord. All of these axons decussate, that is, cross over to the contralateral side of the spinal cord. (They thus differ from the axons in the archispinothalamic and paleospinothalamic pathways, which make bilateral connections to the brain structures that they innervate, because some of their collaterals do not decussate and instead ascend directly on the same side.) Once they decussate, the axons of the spinothalamic pathway continue their ascent on the contralateral side, through the anterior lateral part of the spinal cord (which is why this pathway is also sometimes called the anterolateral or ventrolateral tract). The axons then enter the medulla oblongata, where they are joined by the axons of the trigeminal spinal nucleus, which convey pain sensations from the face (see sidebar), as well as by the medial lemniscal pathway, which is responsible for the sense of touch. Most of the fibres in the neospinothalamic tract that come from parts of the body below the neck terminate in the posterior ventrolateral nucleus of the thalamus. The neurons in this nucleus are the third in the neospinothalamic pathway and send their axons to the primary somatosensory cortex.
|
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
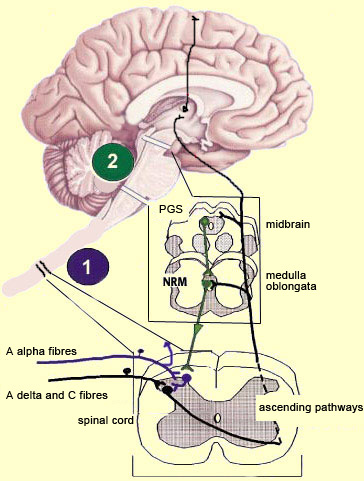
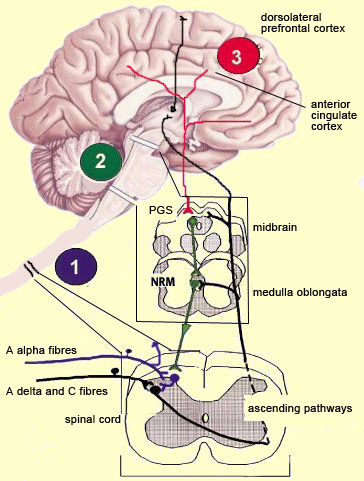
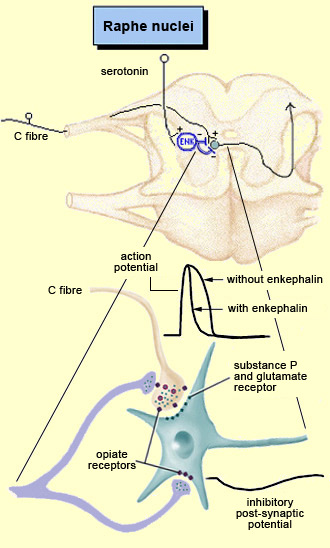
The brain is not a passive receiver of sensory messages, but rather a centre that interprets them and makes constant adjustments accordingly. For example, everyone knows that the way you perceive pain will be influenced by whether you focus on it or think of something else instead. And it seems reasonable to suppose that evolutionary selection may have favoured those individuals who could ignore pain signals for long enough to take actions that let them escape and survive danger. What interests neuroscientists is just how people manage to control and attenuate their perception of pain. The mechanisms for the descending control of pain were first described in detail in relation to the spinal cord, by Melzack and Wall’s gate theory. This theory marked the end of the conception of pain as a simple, primitive alarm system, by showing that the transmission of pain signals was controlled in some ways in the spinal cord and hence possibly at various locations in the brain as well. Thus the eminently subject nature of pain, and the many psychological factors that can affect it, were found to have a neural substrate. The neural pathways originating in the higher brain centres that generate these psychological states exert their influence on subcortical areas and are hence by definition descending pathways. These pathways have an inhibitory effect that, depending on how much it is activated, can “close the gates” to various degrees in the centres that relay pain signals up the ascending nociceptive pathways.
For example, the diffuse noxious inhibitory controls induced by pain stimuli originate in the brainstem. These control mechanisms are often referred to by their acronym, DNICs, and scientists have known about them for a long time, because they are the means by which one pain masks another. In ancient times, burning-hot metal tips were applied to various points on patients’ bodies to relieve certain kinds of pain elsewhere. In modern times, when cattle ranchers need to carry out painful procedures such as castration, they may apply a clamp to the animals’s nose to mask the pain. In other words, when two pain stimuli are applied to two different areas of the body that are distant from each other, perception of the weaker of the two stimuli is inhibited. In the late 1970s, a formal hypothesis was developed to explain this phenomenon: specifically, that a localized nociceptive stimulus can result in a generalized inhibition of afferent nociceptive nerves elsewhere in the body. For example, researchers have conducted experiments in which they applied a pain stimulus to the tip of a rat’s paw and then monitored the electrical activity in a nociceptive neuron in the spinal cord that was responding to this stimulus. The researchers then found that they could inhibit this nociceptive response by applying pain stimuli to numerous points elsewhere on the rat’s body. The researchers also found that non-nociceptive stimuli were totally ineffective in this regard, and that the degree of inhibition was proportional to the intensity and duration of the pain stimulus applied to inhibit the nociceptive response.
The periventricular grey matter and its continuation, the periaqueductal grey matter, which is located around the cerebral aqueduct in the midbrain, have been studied extensively (see sidebar). It is now known that both structures not only send descending projections to the spinal cord and the cerebellum, but also send ascending projections to the thalamus and the frontal lobes. Neuroscientists therefore believe that this periventricular and periaqueductal area may modulate pain both centrally and in the spinal cord.
|
| |
|
|
|
|
|
|
|
|