|
|

 |
 |
 |

 |
 |
In 2004, U.S. neuroscientist A.
Vania Apkarian used magnetic resonance imaging to compare the brains of healthy
persons with those of people suffering from chronic back pain. In the latter group,
he observed a thinning
of the grey matter in the brain comparable to the loss of grey matter
observed in 10 to 20 years of aging. And the longer these people had been living
with this chronic pain, the greater the volume of grey matter they had lost.
This loss was especially evident in the thalamus and the prefrontal cortex, an
area associated with problem-solving. This finding was consistent with Apkarian’s
earlier observation that people with chronic pain took longer to solve certain
mental-skill-testing problems than healthy people did. Scientists are well
aware of the
harmful effects of stress on certain neurons in the brain that are particularly
involved in memory. But it is still hard to say whether this stress is directly
responsible for the thinning of the grey matter, or whether the stress is instead
the source of chronic pain, which then in turn causes the reduction in brain volume.
|
Studies have shown that the nucleus
accumbens, a key part of the brain’s reward
circuit, is activated during certain experiments that employ nociceptive stimuli.
Moreover, in many cases this activation appears to be associated with a variation
in the level of endorphins
in the vicinity of the nucleus accumbens. The fact that dopamine
is also involved in the analgesia produced by the placebo
effect is consistent with this finding, because the neurons of the nucleus
accumbens are highly sensitive to this neurotransmitter, which they receive from
the ventral
tegmental area. These findings tend to support the hypothesis that
there are specific physiological mechanisms behind what we subjectively perceive
as a continuum, from the onset of pain to its subsidence and
then on to pleasant and highly pleasant sensations. This same idea that pain
and pleasure are part of a single spectrum can be found in the writings
of philosophers from past centuries, such as Spinoza
and Bentham.
| | |
The
scientific search for a single “pain centre” in the brain has proven
fruitless. If such a centre existed, then millions of people might be relieved
of their chronic
pain by treatments to remove this centre surgically or neutralize it chemically.
But no such centre has been found. Pain is a subjective phenomenon with multiple
dimensions, some discriminative, some affective, and others cognitive, so it is
no surprise that science has shown that any given sensation of pain is actually
produced by the interactions of a network
of brain structures that are activated by a particular nociceptive stimulus.
Science has also shown that the activity of this network
is highly sensitive to “top-down”
regulatory processes, which would explain phenomena such as the placebo
effect. The way we experience a given source of pain is also influenced by
our personal experience and our cultural
heritage, which means that an even broader range of brain structures are involved.
That said, neuroscientists do now acknowledge that there is at least a partial
degree of functional specialization among the brain structures involved in the
various components of pain. Researchers are now attempting to associate different
subsets of brain structures with these different components of pain and thereby
propose an overall working model of pain. Given the complexity of the phenomenon
that these models are supposed to represent, they are still the subject of much
lively debate. Broadly speaking, the underlying concept
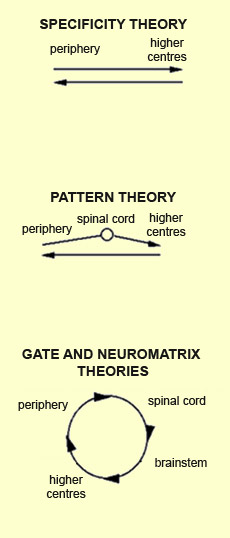
of theories of pain has evolved over time from one of linear causality to one
of circular causality. In the early theory of intensity, pain
was deemed to result from the excess activity of certain nerves that were not
necessarily pain-specific. Then, in the 17th century, René
Descartes was one of the first authors to discuss pain as a specific sense,
just like the senses of sight,
hearing, and smell. In 1894, Von Frey stated an explicit
theory of the specificity of sensations. According to this theory,
the type of nerve ending determines the nature and quality of the sensation perceived.
The resulting information then travels basically from the periphery to the higher
centres, where it reaches something like a “pain centre”, then travels
back downward as motor control information, without having been altered in any
significant way. Thus this theory does not allow for any possible changes of psychological
origin, such as might result from attention
or from past experiences that give a particular meaning to a particular situation.
In this model, the brain and the subcortical relays are nothing more than passive
receptors.
Source: Charest, Lavignolle, Chenard, Provencher and
Marchand, “École interactionnelle du dos”, Rhumatologie,
46, 221-237, (1994)
| | Unable
to provide suitable explanations for phenomena such as chronic pain, specificity
theory subsequently gave way to various pattern theories of pain.
These theories added to the linear ascending pathway various relays that begin
the process of integrating the activity of nerve fibres that have different receptive
properties. Such integration would take place, for example, in the gelatinous
substance of the spinal cord, the ventral posterior nuclei of the thalamus,
and the somatosensory cortex. Motor control signals would then be returned downward
in linear fashion. The development of the gate
theory of pain starting in the 1960s, and of neuromatrix
theory after that, was based on the finding that pain results from a
multitude of interactions and information exchanges at several levels in the nervous
system. The ascending nociceptive information is modulated at each of these multiple
relays before it is integrated into a perception of pain. Perhaps the chief advantage
of this circular model of pain is that it provides a better explanation of how
the nociceptive, discriminative, affective, and behavioural components of pain
can all influence one another. | The
concept of the neuromatrix of pain was first advanced by Canadian psychologist
Ronald Melzack, in the late 1980s, in an attempt to explain the strange but very
common phenomenon of “phantom
limb” pain, in which people who have had a limb amputated feel very
real pain that seems to be coming from that limb. This phenomenon clearly shows
that pain is not generated by a one-way system. Melzack’s proposed explanation
was that pain is actually generated by neural activity in a network composed of
several different structures in the brain, and that this network can generate
pain even when there is no sensory stimulus to trigger it.
In the case of phantom-limb pain, Melzack proposed, the conflict between the visual
feedback that the limb is absent and proprioceptive representations that it is
present might cause confusion in the neuromatrix, and this confusion would then
generate the pain. Evidence in support of this hypothesis has been provided by
experiments in which mirrors were used to give amputees with phantom-limb pain
the visual illusion that their amputated limbs were still present. In some cases,
this measure was effective in relieving the phantom-limb pain.
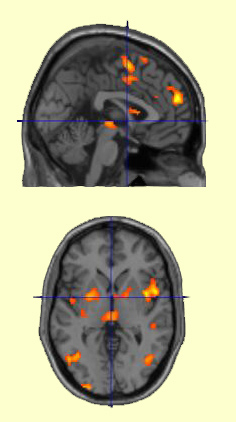
Activation
of structures of the pain neuromatrix, including the insula, the anterior cingulate
cortex, the periaqueductal grey matter, the medial prefrontal cortex, and the
supplementary motor area | | This
neuromatrix, or pain matrix, thus consists of all the parts of the brain whose
activity fluctuates when an individual is experiencing pain—a vast neural
space in which various,
distinctive types of pain can be encoded. Each of these types of pain has
what Melzack calls its own special neurosignature: a unique activation
pattern of the neuromatrix or of some subset of it. (Other scientists use the
term neuronal
assembly to describe this kind of association of neurons.) And because the
details of the connections in the brain of each individual are different, each
individual’s neurosignatures are necessarily different too. Likewise, because
synaptic connections can be modified by experience, any given neurosignature
in a given individual’s brain will change structurally with the passage
of time. | To account for all the
different facets of the phenomenon of phantom pain in amputees, Melzack proposed
a neuromatrix comprising numerous
brain structures involved variously in the discriminatory, affective, cognitive,
and motor aspects of this experience. Melzack’s proposed neuromatrix included
at least three major neural circuits whose importance has been confirmed by the
numerous brain-imaging studies that followed. The first is a lateral spinothalamic
ascending nociceptive pathway, which performs a discriminative function
and includes the ventral posterior nuclei of the thalamus and the somatosensory
cortex. The second is a medial spinothalamic pathway, which has a more affective
and motivational function and involves the brainstem, the ventral medial
nuclei of the thalamus, the limbic system, and the frontal cortex. The third circuit
involves the associative areas of the inferior parietal cortex.
Subsequent research has shown that this neuromatrix
also involves other parts of the brain, such as the orbitofrontal cortex, the
prefrontal cortex (Brodmann areas 9, 10, and 44), and the motor cortex (for example,
Brodmann area 6 and the supplementary
motor cortex), as well as certain regions of the midbrain,
such as the periaqueductal
grey matter and the lenticular (or lentiform) nucleus. Many
neuroscientists have even come to regard structures such as the anterior
cingulate cortex and the insula as key areas whose activation necessarily
accompanies certain aspects of pain, and particularly its affective component.
Without reverting to a description of these areas as “pain centres”,
these scientists do note that their neurons show a great deal of specificity to
certain aspects of pain. This shows that the pain neuromatrix may include various
nodes, and that the activity of some of these nodes may be more significant than
that of certain others.
|
|