|


Brain
Rhythms: The Oscillations That Bind
The recording of an
EEG is a completely pain-free, non-invasive procedure,
because the electrodes are simply glued to the patient’s
scalp. The various traces in an EEG represent the differences
in electrical potential detected between these various
electrodes.
Where do these differences come from? Mainly from the
differences in the activity levels of the neurons in
the various
layers of the cerebral cortex when different
areas of the cortex are compared. But the contribution
of each individual neuron to the resulting signal is
extremely small. Also, to reach the electrodes, this
signal must pass through several layers of tissue, including
the meninges,
the skull, and the scalp, which attenuate
this signal considerably. The signal will not be strong
enough for the electroencephalograph to pick up unless
several thousand neurons are firing simultaneously. |
| |
| HOW
NEURONS GENERATE BRAIN WAVES |
|
The electroencephalograph (follow
the Tool module link to the left) is an instrument that provides
information about the overall activity of large groups of neurons
in the brain. The print-out from an electroencephalograph is
called an electroencephalogram, or EEG. An EEG will never tell
you what someone is thinking, but it will tell you whether they are thinking,
or whether they are simply awake, or, for that matter, asleep.
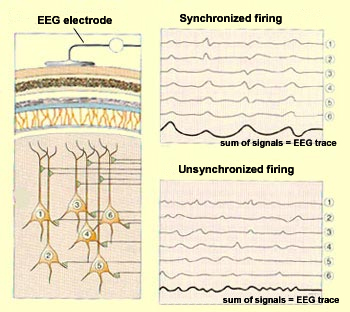
Each electroencephalograph sensor consists
of an electrode that is attached to a particular location on
the scalp and that picks up a signal from the neurons in a given
area of the cortex. The more synchronized the activity of these
neurons, the greater the amplitude of the signal that the sensor
receives. And the greater the amplitude of this signal, the greater
the deflection (movement) of the pen that records the EEG trace.
Adapted from: Neurosciences,
Bear, Connors, and Paradiso, Éditions Pradel, 2002 |
When a group of cortical
neurons are excited simultaneously (that is, when all their dendrites receive
stimuli at the same time), their weak individual signals
are summed so that they become perceptible to the electrode
attached to the adjacent portion of the scalp. Conversely,
when the stimuli received by these neurons are not synchronized,
their summed signals are weaker, and the amplitude of the
resulting EEG trace will be smaller and more irregular. |
Broadly speaking, when the cortex is busy analyzing information
from a sensory stimulus or internal process, the activity of
its neurons is relatively high but also relatively unsynchronized.
Each little group of neurons is being activated by a different
aspect of the cognitive task that the cortex is performing, so
the activity of these groups is not highly synchronized, and
the amplitude of the EEG trace is correspondingly small. In this
state, high-frequency beta
waves predominate.
In contrast, during non-REM sleep, the cortical neurons are
no longer busy processing information. In addition, many of them
are being stimulated by the
same slow, rhythmic pulse from the thalamus, so their activity
is highly synchronized. As a result, the EEG shows the high-amplitude,
low-frequency trace characteristic of delta
waves.
But what is the source of this rhythmic
brain activity? Research indicates that the brain may synchronize
these periodic oscillations in two different ways. In some cases,
a group of neurons may be activated synchronously because all
of them are influenced by a single generator, or pacemaker. In
other cases, a group of neurons may set their own pace,
by exciting or inhibiting one another.
If we compare groups
of neurons to groups of musicians, we can say that in the
first case, the neurons are like the members of an orchestra,
all following instructions from the same conductor. In
the second case, they are more like jazz musicians at a
jam session, each constantly adjusting to the others by
listening to and watching them.
Another good analogy for the second of these synchronization
mechanisms is something that often happens at the end of
a concert: the members of the audience start calling for
an encore and then spontaneously start clapping their hands
rhythmically, in unison. No one has to help them co-ordinate
their clapping. People can clap their hands only within a
narrow frequency range, so once a few people start clapping,
it is easy for others to hear their rhythm, start clapping
themselves, and then speed up or slow down until they are
in phase with it. |
 Source: Neurosciences,
Bear, Connors, and Paradiso, Éditions Pradel,
2002
Source: Neurosciences,
Bear, Connors, and Paradiso, Éditions Pradel,
2002 |
A network of neurons interact in somewhat the same way, but
through excitatory and inhibitory connections, rather than sight
or sound. And because these neurons can generate action
potentials only within a limited frequency range, though
these potentials may be only partly synchronized at first, they
can become more so until they develop major rhythmic oscillations.
| |