|
|

 |
 |
 |

 |
 |
Though the frequency
of the nerve impulses of the neurons of the suprachiasmatic
nuclei is the phenomenon that expresses their rhythmicity,
these impulses are not necessary to generate this rhythm.
Like a watch whose hands have been temporarily removed, the
mechanism that generates the endogenous rhythm of these neurons
continues to function even when they are isolated from one
another in culture media.
Also, when tetrodoxin (TTX) is applied
to these neurons so as to block their sodium
channels, it prevents them from producing action potentials
but in no way affects the rhythm of their activity. Moreover,
when the TTX is removed, the action potentials resume with
the same phase and the same frequency as before.
Like the hands of a clock, the action
potentials generated by the neurons of the human biological
clock enable it to tell what time it is, but not to keep
track of how much time has elapsed. It is rather at the molecular
level, within the genes, that the
most fundamental mechanism of this biological clock resides. |
|
|
| LIGHT-SENSITIVE
GANGLION CELLS |
|
A pair of small areas in the hypothalamus, the
suprachiasmatic nuclei, are recognized as constituting a
central clock that co-ordinates the cyclical variations in several
functions of the human body, such as the sleep cycle
and the cyclical secretion of hormones.
The discharge frequency of the cells of the
suprachiasmatic nuclei varies according to a regular 24-hour cycle.
This rhythmic activity is not the
result of communication among the neurons of these nuclei,
but rather of feedback
loops inside each of these cells.
Scientists reached this conclusion after
removing neurons from the suprachiasmatic nuclei of rats and isolating
these cells in a culture medium in vitro, where they had
no connection with one another. The researchers observed that the
activity of each individual neuron continued to vary in a cycle
lasting about 24 hours (see sidebar to the left).
But unlike suprachiasmatic nucleus cells
in the brain, which synchronize their activity with the day/night
cycle, suprachiasmatic nucleus cells in vitro do not.
Like any other clock, the human body’s biological clock needs
to be reset periodically. For that to happen, every cell in this
clock must resynchronize itself daily with external cues that tell
it when the day begins and ends. These external cues, also known
as Zeitgebers (German for “time givers”),
include the ambient temperature, the consumption of meals, ambient
noise, and the body’s activity level. But the strongest of
these cues is undoubtedly the overall intensity of the ambient
light.
There must therefore be a neural pathway
that leaves the eye from the retina and
transmits the variations in light intensity to the cells of the
biological clock in the suprachiasmatic nuclei. Because unlike
suprachiasmatic nucleus cells in vitro, which are cut
off from any neural connections from the retina, suprachiasmatic
nucleus cells that are still in place in the brain can receive
this information through the optic nerve.
The cells in the retina that detect light
intensity and pass this information on to the suprachiasmatic nuclei
are neither
rods nor cones, but rather certain
ganglion cells that have distinctive properties and that are
dispersed among all the other ganglion cells.
Numerous experiments have confirmed this
hypothesis. For example, we know that people who are blind maintain
a normal biological rhythm. But when people suffer head injuries
that completely destroy both optic nerves, then they lose not only
their sense of vision but also their ability to regulate their
circadian rhythm. Mice whose layer of rods and cones has degenerated
completely also preserve their circadian rhythm. Thus all the evidence
suggests that it is indeed ganglion cells that constitute the first
link in this non-visual light-sensitive system.
In subsequent experiments, various tagging methods have revealed
that this particular sub-population of ganglion
cells does in fact send axons directly to the dendrites of
the neurons of the suprachiasmatic nuclei (see box below).
Source: Ralph Nelson, http://webvision.med.utah.edu
This non-visual system for detecting light
intensity also seems to be involved in controlling the pupillary
reflex (the process by which the pupil of
each eye dilates when the light is too dim and contracts when it
is too bright). Certain axons in the retinohypothalamic tract must
therefore continue their path beyond the hypothalamus to other
cerebral nuclei that are involved in this reflex, such as the lateral
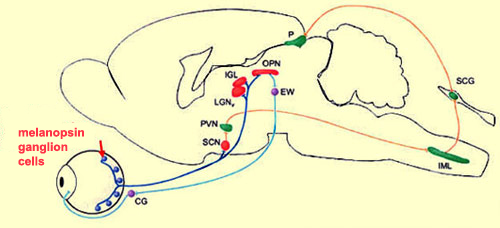
geniculate nucleus, the olivary pretectal nucleus, and the Edinger
Westphal nucleus (respectively LGN, OPN, and EW in the diagram
above).
To identify the targets
of the axons of the ganglion cells involved in detecting
light intensity, researchers have used “knock-in” mice,
in which the tau-lac Z gene has been “knocked
in” to (inserted into) the melanopsin-containing ganglion
cells. This gene produces a protein that can be stained selectively.
And because this protein travels along the axon, its path
and its various destinations are thereby revealed.
These experiments showed that the suprachiasmatic nucleus was
very densely innervated by the axons of the ganglion cells
that produce melanopsin. But several other parts of the brain
also receive connections from these cells, in particular, the
nuclei involved in the pupillary reflex (as the above illustration
shows).
|
|
|





